Blakiston's Fish Owl Natural History
Excerpted from an early revision of the manuscript:
Slaght, J.C., and S.G. Surmach. 2008. Biology and conservation of Blakiston's fish owls in Russia: a review of the primary literature and assessment of the secondary literature. Journal of Raptor Research 42:29-37. Full citations of all references are here.
1. Distribution
2. Population Size
3. Habitat, Breeding, Behavior
4. Prey Base and Hunting
5. Vocalizations
6. Conservation Threats
Blakiston's fish owls have a fragmented distribution in the Russian Far East, northern Japan , and northeastern China. There are two generally-recognized subspecies, an island subspecies, which occurs on Hokkaido Island, Japan, and Kunashir and Shikotan Islands of the southern Kuril Islands, Russia (Dykhan and Kisleiko 1988, Brazil and Yamamoto 1989, Takenaka 1998, Berzan 2005), and a more broadly-distributed mainland subspecies, which ranges from the Russian provinces Magadan in the north to Primorye in the south (Surmach 1998). The species may occur in North Korea ; however political tensions have prevented recent survey attempts there (W. Duckworth pers. comm.).
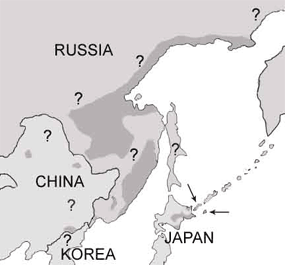
Of the <60 breeding pairs (150-205 birds total) that comprise the island subspecies, ~30-35 pairs (80-120 individuals) live on Hokkaido Island (Brazil and Yamamoto 1989, Takenaka 1998), with the remaining ~20-25 pairs (70-85 individuals) primarily on Kunashir Island (Dykhan and Kisleiko 1988, Berzan 2005). There have been recent detections on Shikotan Island , but breeding has not been confirmed (Grigorev 2005).
The population of the mainland subspecies is more difficult to quantify because of insufficient surveys across such a vast area of potential range. Population numbers for all Russia (mainland and island subspecies combined) were described at 300-400 pairs in the early 1980s (Pererva 1984), an estimate that is the basis for reports in the English-language secondary literature (Collar and Andrew 1988, Collar et al. 1994, del Hoyo et al. 1999, König et al. 1999, Stattersfield and Capper 2000). Surmach (1998) suggests that the population in the southern Russian Far East (encompassing all Primorye and Khabarovskii Krai south from the Amur River) is approximately 100-130 pairs based on his surveys. With extrapolation to the entire fish owl range, the population could be >800 pairs (S. Surmach, in Collar 2001).
Recent surveys by Surmach (2006) estimate one pair of Blakiston's fish owls every 3.8 river km along the Samarga River in northern Primorye, possibly the highest natural concentration of this species globally. In the secondary literature, concentrations of breeding pairs in suitable habitat are generally described as one pair every 6-12 river km (Pererva 1984, Voous 1988, Hume 1991, Collar et al. 1994, del Hoyo et al. 1999, König et al. 1999).
3. Habitat, Breeding, and Behavior
Blakiston's fish owls require cavernous old-growth tree cavities for suitable nest sites and stretches of productive rivers that remain at least partially unfrozen in winter (Yakovlev 1929, Vorobev 1954, Shibnev 1963, Pukinskii 1973, Dykhan and Kisleiko 1988, Dugintsov and Teryoshkin 2005). Of the 23 nest tree descriptions in the Russian literature, eight are elm ( Ulmus sp.), five are Japanese poplar ( Populus maximowiczii ), four are willow ( Salix sp.), two are chosenia ( Chosenia arbutifolia ), two are Mongolian oak ( Quercus mongolica ), one is ash ( Sorbus sp.), and one is stone birch ( Betula ermanii ). Nest types are cavity nests ( N= 12), broken-top nests ( N= 7), tree fork nests ( N= 1), and unspecified ( N= 3). Nest height range is 2-18 m (Nechaev and Kurenkov 1986, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988, Takenaka 1998, Pukinskii 1973, 1993, Dugintsov and Teryoshkin 2005, Yelsukov 2005, S. Surmach unpubl. data). Some secondary sources (Dementev 1951, Fogden 1973, Sayers 1976, del Hoyo et al. 1999, König et al. 1999) write that Blakiston's fish owls nest on the ground, but there are no such records in the primary Russian literature (although there is a recent record of Blakiston's fish owls nesting on a cliff in Japan; Takenaka 1998).
In addition to old-growth trees capable of providing nest cavities, access to open water in winter is another important consideration for Blakiston's fish owls (Yalovlev 1929, Spangenberg 1948, Surmach 1998). Open water is found only where the current is sufficiently fast-flowing or there is an upwelling of warm spring water (Surmach 1998). Dugintsov and Teryoshkin (2005) report that such areas average every 5-7 km on the Selemdzha River . Surmach (1998) suggests that slower-moving streams are more important to Blakiston's fish owls than the main river channels, and that, given sufficient prey, openings as small as a few m 2 are sufficient to sustain a pair of resident owls through the winter.
Blakiston's fish owls can form pair bonds as early as their second yr, and reach sexual maturity by age 3 (Pukinskii 1973). It is not clear what dictates breeding attempts, as the species does not necessarily breed every yr (Pukinskii 1973, Berzan 2000, Dugintsov and Teryoshkin 2005, S. Surmach unpubl. data). Breeding is likely tied to environmental and other factors not yet identified. Courtship occurs from January-February, with a clutch of one or two eggs laid in March (Nechaev 1969, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988). The female incubates the clutch while the male brings food to the nest (Brazil 1985, S. Surmach, pers. obs.) Once chicks hatch, the female continues to warm them during the day, but joins the male to bring food to the nest at night (J. Slaght, pers. obs.). Young fledge up to 50 d post-hatching (Pukinskii 1993). Data on breeding success are scant: on Kunashir Island during a 6 yr period breeding success was 24%; with six fledglings resulting from 25 eggs (Berzan 2000). Juveniles remain on their natal territory into their second yr, apparently dispersing as late as July the following yr (Pukinskii 1973). This unusually long pre-dispersal period may be why Shibnev (1963) interprets several full-grown fish owls in a single tree in April as evidence of gregarious behavior, and is repeated as such by Voous (1988). However, Pukinskii (1973) considers this a misunderstanding of fish owl biology.
An observation by Pukinskii (1973) of congregations of five-six birds at open-water areas is also misinterpreted by several secondary sources (Voous 1988, Hume 1991) as evidence of gregarious behavior. The original text clearly identifies such congregations as rare events in unseasonably cold winters, when regular foraging habitat is presumably ice-covered and therefore inaccessible.
Both Pukinskii (1973) and Mikhailov and Shibnev (1998) stated that fish owls are non-migratory. Although Pukinskii (1973) dismissed a description by Shibnev (1963) of apparent fish owl migration in the Bikin River basin in winter, several secondary sources have cited it (Voous 1988, König et. al. 1999). At present however, there are few data to verify either assertion, as fish owl seasonal movements are poorly understood. Although long-distance migration is unlikely (Pukinskii 1973, Mikhailov and Shibnev 1998, S. Surmach unpubl. data), short distance movements, such as seasonal shifts in home range, may occur and should be investigated.
Several sources link Blakiston's fish owl distribution to rivers rich with salmonid fish (Pukinskii 1973, Dykhan and Kisleiko 1988), but it appears that fish owls can persist on smaller prey species as well (Surmach 1998). Driven by seasonal and spatial availability, fish owl prey base is actually quite diverse: in addition to fish, direct observation or examination of prey remains shows predation of a variety of waterfowl species, small mammals, and amphibians (Vorobev 1954, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005). Reliance on certain prey species is seasonal: in spring, frogs ( Rana sp.) are particularly important and taken in great abundance (Pukinskii 1973). Interestingly, although many secondary sources (Fogden 1973, 1992, Flint et al. 1984, König et al. 1999, Collar 2001) list crayfish as an important food source, the most recent documentation of crayfish predation on the mainland is by Pukinskii (1973), with the only other report from Yakovlev (1929), although Voronov and Zdorikov (1988) describe finding crustacean remains in fish owl pellets on Kunashir Island. A similar report by Pererva (1984) erroneously cites Dementev (1951) as a reference, although this information is not found in that publication. Anecdotal reports of crayfish population declines in Primorye over the last several decades may account for their apparent disappearance from fish owl diet on the mainland.
The most commonly described hunting technique used by fish owls is dropping on prey in shallow water, often from a low, nearby perch, such as a snag or rock, or in winter simply from the ice edge (Yakovlev 1929, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005). Upon identifying prey, fish owls either drop directly into the shallow water or sail a short distance (Yakovlev 1929, Pukinskii 1973, Dykhan and Kisleiko 1988, Voronov and Zdorikov 1988). Fish owls generally take frogs and other smaller prey directly back to a survey perch or another roost to be consumed. Larger prey such as fish and waterfowl are partially consumed on the riverbank or ice edge before being taken to a habitual roost to be finished (Yakovlev 1929, Pukinksii 1973, Voronov and Zdorikov 1988, Dugintsov and Teryoshkin 2005).
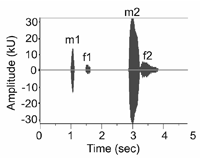 Pukinskii (1974) describes a variety of Blakiston's fish owl vocalizations, perhaps the most interesting of which is the duet. Described vaguely as "elaborate" (Hume 1991, del Hoyo et al. 1999, König et al. 1999) and "complicated" (Voous 1988) in the secondary literature, duets are actually quite simple, although structure differs between the mainland and island subspecies. On the mainland, a duet consists of four distinct notes, where the male produces the first and third notes, and the female produces the second and fourth notes (Fig. 2). In contrast, an island subspecies duet is only three notes, with the male producing a two-note call followed immediately by a single note from the female (Brazil and Yamamoto 1989). Duets are occasionally reversed when the pair is agitated, with the female initiating the call and the male responding (Pukinskii 1974, J. Slaght pers. obs.).
Pukinskii (1974) describes a variety of Blakiston's fish owl vocalizations, perhaps the most interesting of which is the duet. Described vaguely as "elaborate" (Hume 1991, del Hoyo et al. 1999, König et al. 1999) and "complicated" (Voous 1988) in the secondary literature, duets are actually quite simple, although structure differs between the mainland and island subspecies. On the mainland, a duet consists of four distinct notes, where the male produces the first and third notes, and the female produces the second and fourth notes (Fig. 2). In contrast, an island subspecies duet is only three notes, with the male producing a two-note call followed immediately by a single note from the female (Brazil and Yamamoto 1989). Duets are occasionally reversed when the pair is agitated, with the female initiating the call and the male responding (Pukinskii 1974, J. Slaght pers. obs.).
Although other sources of mortality may remain undetected due to lack of study, most known Blakiston's fish owl mortality is due to contact with humans (all but two records; Pukinskii 1993, Yelsukov 2005). Of 12 cases of fish owl mortality known to Yelsukov (2005), all but three birds were shot by hunters. Surmach (1998) reports 10 cases of fish owls shot wantonly, and two birds shot for commercial gain (i.e., sale of live-mount specimens). In winter fish owls are accidentally caught in furbearer snare lines (Vorobev 1954, Dykhan and Kisleiko 1988, Averin and Antonov 2005, Yelsukov 2005). Surmach (1998) believes fish owls can survive 3-5 d in such snares, and are often healthy enough to be released once found, but fish owls were killed by the trapper in 41 of 48 known cases of trapping. Education of local populations may therefore be a key conservation tool (Pererva 1984, Surmach 1998).
Direct hunting of fish owls, although not common, should also be considered a threat. The native Udege peoples of the Bikin and Samarga river basins target the owls in winter as a food source (Vorobev 1954, Mikhailov and Shibnev 1998). Meise (1933) reports similar findings from Manchuria . Spangenberg (1965) describes encounters along the Iman River in 1938-39 with Udege who use dried fish owl wings as fans to dissipate biting insect swarms. Recent interviews with Udege in the Samarga River basin indicate fish owl hunting is not as widespread as it once may have been (J. Slaght pers. obs.).
Logging is a considerable threat to Blakiston's fish owl conservation. Although in theory Russian law protects riparian areas from harvest, as logging within 5 km of either bank of major waterways is illegal (Surmach 1998), riparian areas are still being impacted by logging either directly or indirectly. First, the law is not always enforced in remote areas of the Russian Far East, and loopholes in the law are often exploited (Newell and Lebedev 2000). Riparian areas are also impacted indirectly by the creation of roads to facilitate resource extraction (Slaght 2005). Old-growth Japanese poplar—a favored fish owl nest tree species—is chosen by loggers to create makeshift bridges across waterways (J. Slaght pers. obs.). Ash and Mongolian oak, also important fish owl nest tree species, are valued in the timber industry and removed illegally in high volume (Newell and Lebedev 2000). Furthermore, logging roads often remain accessible after an area has been harvested, which facilitates illegal logging in riparian areas (Vandergert and Newell 2003). Following an example from Japan where artificial nest boxes were used to restore nesting opportunities in logged forest (Brazil 1985), nest boxes were constructed on Kunashir Island after natural senescence of nest trees was found to cause nest tree abandonment (Berzan 2000, Grigorev 2005). In both cases, the owls readily utilized the artificial nests and successfully fledged young.
The notion that Blakiston's fish owls are intolerant of human encroachment is prevalent in the secondary literature (i.e. Voous 1988, Hume 1991, del Hoyo et al. 1999, Collar 2001). However, this may not be a wholly applicable generalization (Surmach 1998). First, not all of these reports are credible: Collar (2001) cites Vaskovskii (1956) when noting fish owl displacement in Magadan due to increased human activity. However the original text indicates the opposite, that fish owls are observed there with some regularity. In fact, there are numerous records of fish owls found close to human settlements and even nesting in proximity to villages (Bergman 1935, Gizenko 1955, Pukinskii 1973, Tarkhov and Potapov 1986, Shokhrin 2005, S. Surmach unpubl. data). Furthermore, we know of several successful fish owl nests located near human foot trails, and a pair of owls that nested in a forest selectively logged several decades ago. From a conservation perspective, all of these observations suggest an encouraging level of tolerance and adaptability by the species (Surmach 1998), although more study is needed to evaluate reproductive success and survivorship of individuals living near human settlements.